Deciphering the history of human-induced ocean acidification recorded in coral reefs
Summary of AIST Press Release on August 21, 2017
>>Japanese
One third of the anthropogenic carbon dioxide, which has been increasing since the Industrial Revolution, dissolves into the ocean, decreasing pH in seawater. It has been concerned that the ocean acidification, which can inhibit the calcification of marine organisms, may have harmful effects not only on marine ecosystem but also on our economic activity.
The research group consisting of the University of Tokyo, Japan Agency for Marine-Earth Science and Technology (JAMSTEC), the Geological Survey of Japan, and the Meteorological Research Institute has succeeded in high-precision analyses of boron and carbon isotopes in the skeleton of massive Porites corals, and deciphered the one-hundred-year history of ocean acidification recorded in the skeleton. The results indicate that decreasing seawater pH may have caused the decline in pH of coral calcification fluid and that the ocean acidification may have given negative influence on the calcification of corals. The finding is crucial in estimating the future of coral ecosystem.
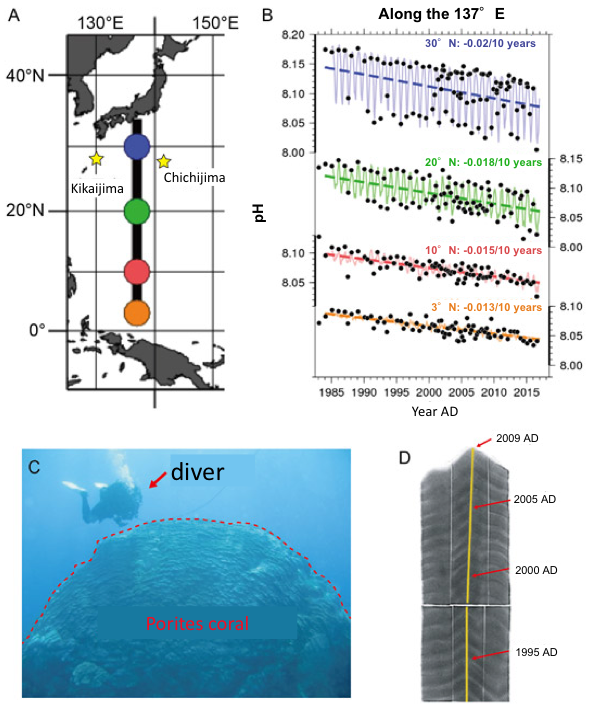
Figure 1. A: Location of Chichijima and Kikaijima Islands, B: Interannual variation of seawater pH along the 137ºE meridian in the western North Pacific. Data are from the Japan Meteorological Agency (http://www.data.jma.go.jp/gmd/kaiyou/shindan/a_3/pHtrend/pH-trend.html), C: 430-year-old massive Porites coral in Kikaijima, D: X-ray photograph along its maximum growth axis (yellow line).
The age is accurately determined by counting of its annual growth bands.
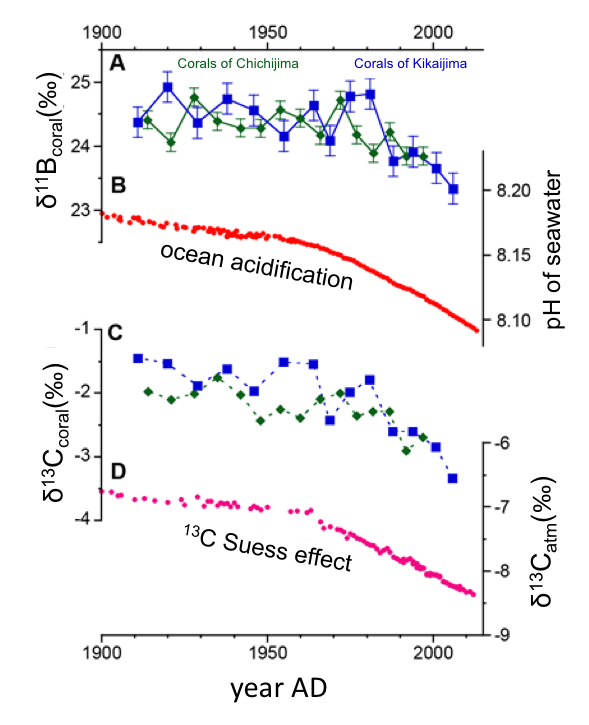
Figure 2. A, C: Coral δ11B and δ13C records show apparent decrease after the 1960s. B, D: Interannual variability of seawater pH in the western North Pacific and δ13C in the atmospheric carbon dioxide.
Their declining trends are called ocean acidification and the 13C Suess effect, respectively.
Publication details
Kubota, K., Y. Yokoyama, T. Ishikawa, A. Suzuki and M. Ishii (2017) Rapid decline in pH of coral calcification fluid due to incorporation of anthropogenic CO2. Scientific Reports, 7:7694, doi:10.1038/s41598-017-07680-0